- About UsOur Story, Our Team & Support Information
- What We DoAdvocacy to Achieve the End of AIDS
- Advance HIV/SRH Integration
- Advocate for Access to High-Impact Prevention
- Improve Research Conduct
- Product Innovation & Availability
- Promote Effective HIV Prevention Policy
- Strengthen Global Advocacy Networks
- Track and Translate the Field
- Our FocusInterventions to End the Epidemic
- ResourcesPublications, Infographics, Events & More
- MediaInformation & Resources for the Press
- Our BlogPrevention News & Perspective
Thursday, September 8, 2016
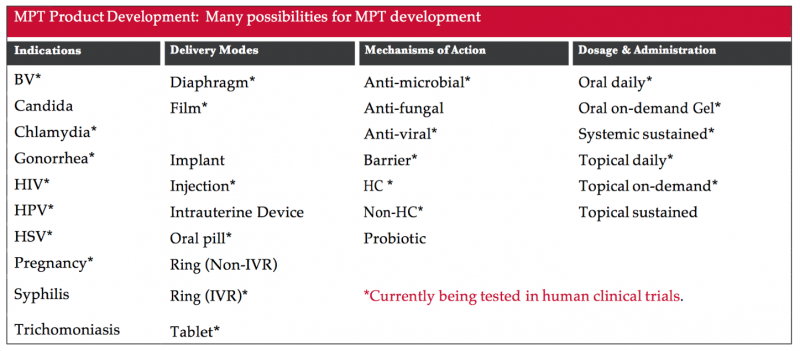
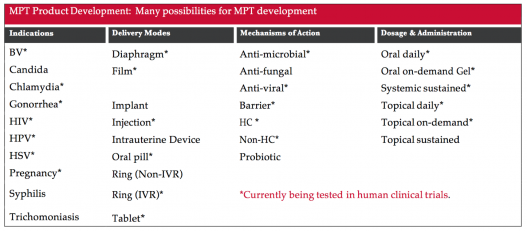
This table shows the indication (prevention effect the product is designed to have—e.g., pregnancy or one or more STIs) the way it will be delivered (e.g., through vaginal gel, pill or injection), how it might work to provide preventive effect (for example, as a physical barrier to prevent fluid exchange or as a hormonal contraceptive) and what kind of dosage could be possible (e.g., daily pill or application, sustained through the blood or around sex).
Excerpted from the multipurpose prevention technologies factsheet.