- About UsOur Story, Our Team & Support Information
- What We DoAdvocacy to Achieve the End of AIDS
- Advance HIV/SRH Integration
- Advocate for Access to High-Impact Prevention
- Improve Research Conduct
- Product Innovation & Availability
- Promote Effective HIV Prevention Policy
- Strengthen Global Advocacy Networks
- Track and Translate the Field
- Our FocusInterventions to End the Epidemic
- ResourcesPublications, Infographics, Events & More
- MediaInformation & Resources for the Press
- Our BlogPrevention News & Perspective
This year's AVAC Report is about the new realities of biomedical HIV prevention research.
 In the last few years we’ve seen major advances, but also have had sobering realizations about the difficulties of developing new HIV prevention options that can succeed both in trials and programs in the real world. Landmark vaccine, microbicide and PrEP trial results energized the biomedical HIV prevention field. Yet, follow-up work from all these trials has been slower than necessary. In the search for new prevention tools for women two recent trials have found very low rates of adherence. These trials have given rise to important questions, not only about women’s willingness to use the test product, but about the research process itself.
In the last few years we’ve seen major advances, but also have had sobering realizations about the difficulties of developing new HIV prevention options that can succeed both in trials and programs in the real world. Landmark vaccine, microbicide and PrEP trial results energized the biomedical HIV prevention field. Yet, follow-up work from all these trials has been slower than necessary. In the search for new prevention tools for women two recent trials have found very low rates of adherence. These trials have given rise to important questions, not only about women’s willingness to use the test product, but about the research process itself.
We argue that the field needs to take a fast, focused look at fundamental assumptions and missed opportunities across the HIV prevention research field—and retool its approaches so that the next generation of research delivers advances that women and men want and will use.
Downloads:
Research Reality Checks
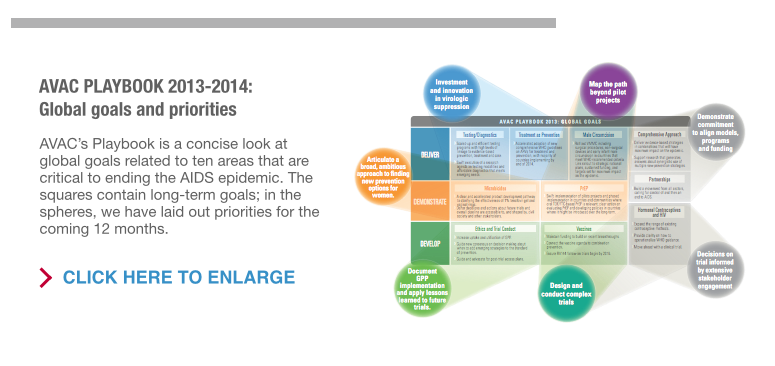
AVAC Report 2013 looks ahead to the coming year with four key recommendations on issues that lie at the intersection of research goals and real-world conditions.
- Launch complex trials to answer complex questions. Clinical trials can seem like a detour from our attempt to control AIDS with the tools available today. This is especially true when the proposed trials are complex and costly—and are part of research agendas that could take years to have a concrete impact. But in many areas, including AIDS vaccines, as well as hormonal contraception and HIV risk, this research is critical and must proceed.
- Map rollout beyond pilot projects. Pilot projects help move clinical research findings into the real world. They are a chance to learn how to deliver a new product. But pilot projects and normative guidance don’t guarantee introduction. In 2014, donors, implementers and national governments need to review progress in pilot projects of daily oral PrEP and non-surgical devices for medical male circumcision—and launch strategic implementation plans where appropriate.
- Invest in innovative approaches to virologic suppression. Simply starting antiretroviral therapy (ART) doesn’t preserve a person’s health or prevent HIV transmission. What matters is sustained treatment and suppression of HIV. Advocates need to make the case for investment in treatment adherence programs, better viral load monitoring in resource-poor settings, and sustained research into new antiretroviral treatments, therapeutic vaccines and functional cures.
- Align programs, models and funding to stay on track to end AIDS. Models are being used to set targets and define core interventions for high-impact prevention in many settings. In 2014, models and programs need to be connected in a feedback loop so that models are informed by research, programs are informed by models and models are improved by real-world experience. This requires sustained funding and visionary leadership at national and international levels.
Refocusing the Women's HIV Prevention Research Agenda
This report’s central focus is the search for female-initiated prevention options. Today, there are only three ongoing efficacy trials of biomedical prevention strategies—and all of them involve vaginal microbicides. These trials are being tracked with interest and concern, in large part because of adherence challenges in some recent studies. Whether positive or not, the results will shape the field. But we cannot wait until the data are in to take action. Now is the time to articulate a broad and ambitious approach to finding new prevention tools for women.
Our Top-Line Recommendations for Women's Prevention Research