- About UsOur Story, Our Team & Support Information
- What We DoAdvocacy to Achieve the End of AIDS
- Advance HIV/SRH Integration
- Advocate for Access to High-Impact Prevention
- Improve Research Conduct
- Product Innovation & Availability
- Promote Effective HIV Prevention Policy
- Strengthen Global Advocacy Networks
- Track and Translate the Field
- Our FocusInterventions to End the Epidemic
- ResourcesPublications, Infographics, Events & More
- MediaInformation & Resources for the Press
- Our BlogPrevention News & Perspective
Monday, April 1, 2019
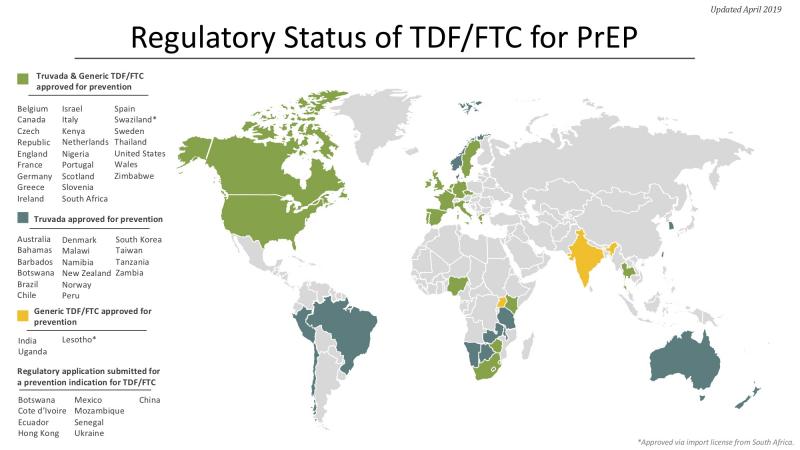
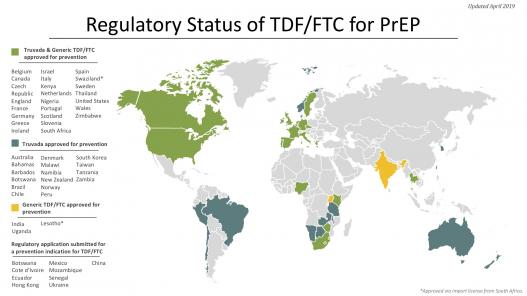
The TDF/FTC combination pill (brand-name Truvada) that has shown efficacy for PrEP is already used for treatment in HIV-positive people, and so is approved and licensed in many countries. One key step for this PrEP strategy is to ensure that the drug is licensed (and therefore available) and that it is approved for use for both prevention and treatment in each country. National guidelines for PrEP use are another key step.