- About UsOur Story, Our Team & Support Information
- What We DoAdvocacy to Achieve the End of AIDS
- Advance HIV/SRH Integration
- Advocate for Access to High-Impact Prevention
- Improve Research Conduct
- Product Innovation & Availability
- Promote Effective HIV Prevention Policy
- Strengthen Global Advocacy Networks
- Track and Translate the Field
- Our FocusInterventions to End the Epidemic
- ResourcesPublications, Infographics, Events & More
- MediaInformation & Resources for the Press
- Our BlogPrevention News & Perspective
Monday, May 18, 2020
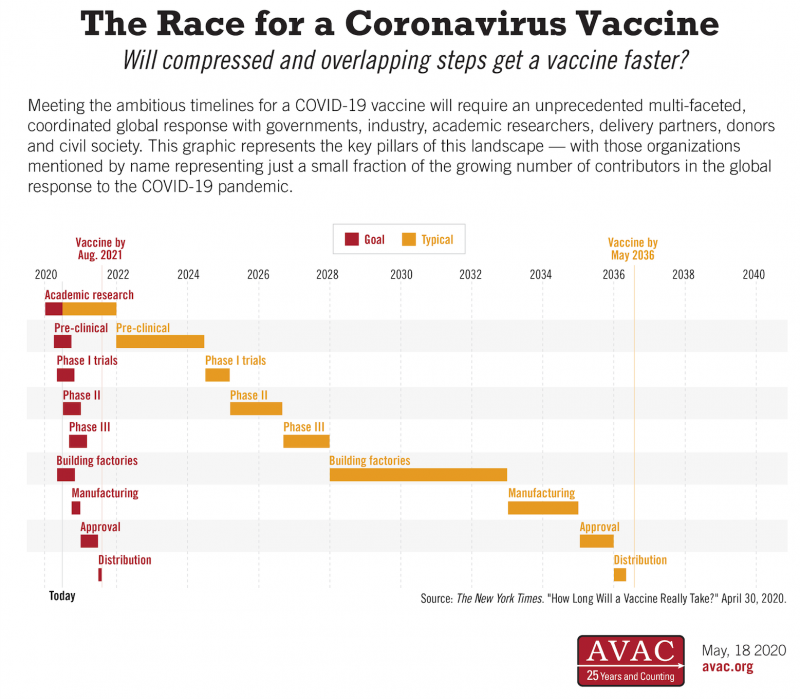
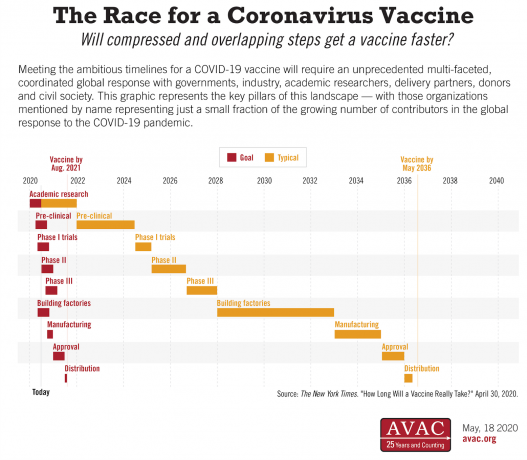
Will compressed and overlapping steps get a vaccine faster? The innovations advocated for in HIV vaccine development that are being employed in the COVID-19 response today include: running certain clinical trials in parallel instead of sequentially; gearing up manufacturing capacity before final study results are in and negotiating public/private commitments in advance to facilitate sustainable access to new vaccines.
Excerpted from Five "P"s to Watch.