- About UsOur Story, Our Team & Support Information
- What We DoAdvocacy to Achieve the End of AIDS
- Advance HIV/SRH Integration
- Advocate for Access to High-Impact Prevention
- Improve Research Conduct
- Product Innovation & Availability
- Promote Effective HIV Prevention Policy
- Strengthen Global Advocacy Networks
- Track and Translate the Field
- Our FocusInterventions to End the Epidemic
- ResourcesPublications, Infographics, Events & More
- MediaInformation & Resources for the Press
- Our BlogPrevention News & Perspective
Thursday, May 18, 2017
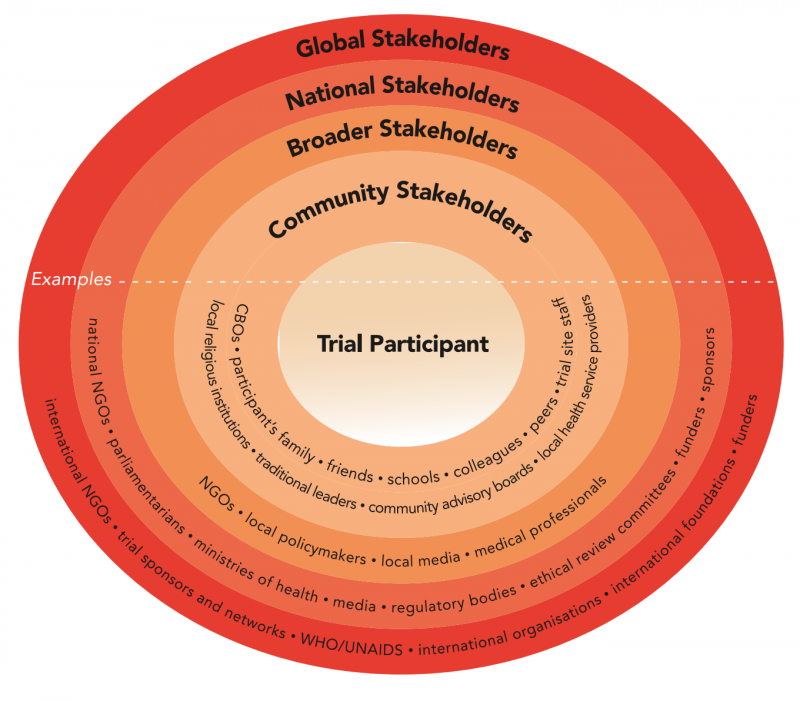
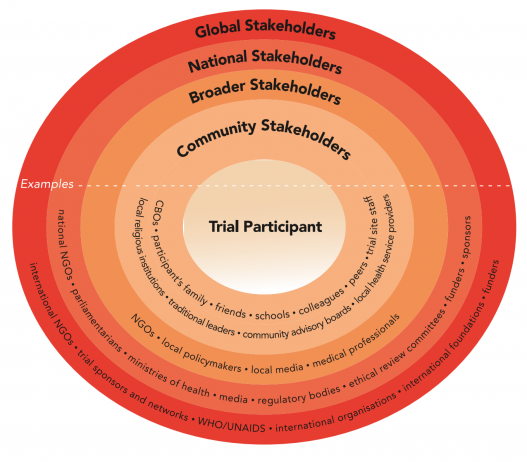
Various stakeholders may influence or be affected by a biomedical HIV prevention trial. Stakeholders include trial participants and other community stakeholders as well as a broader range of national and international stakeholders.
Excerpted from the Good Participatory Practice (GPP) Guidelines, which provide trial funders, sponsors, and implementers with systematic guidance on how to effectively engage with all stakeholders in the design and conduct of biomedical HIV prevention trials. More at avac.org/gpp.